2021 Volume 46 Issue 11 Pages 531-538
2021 Volume 46 Issue 11 Pages 531-538
Developmental and reproductive toxicity (DART) is an important endpoint, and databases (DBs) are essential for evaluating the risk of untested substances using alternative methods. We have constructed a reliable and transparent DART DB, which we named DART NIHS DB, using the publicly available datasets of DART studies of industrial chemicals conducted by Japanese government ministries in accordance with the corresponding OECD test guidelines (OECD TG421 and TG422). This DB is unique because its dataset chemicals have little overlap with those of ToxRefDB, which compiles large-scale DART data, and it is reliable because the included datasets were created after reviewing the individual study reports. In DART NIHS DB, 171 of 404 substances exhibited signs of DART, which occurred during fertility and early embryonic development (49 substances), organogenesis (59 substances), and the perinatal period (161 substances). When the lowest-observed-adverse-effect level (LOAEL) of DART was compared with that of repeated-dose toxicity (RDT), 15 substances (12%) had a lower LOAEL for DART than for RDT. Of these, five substances displayed significant DART at doses of ≤ 50 mg/kg bw/day. The chemical and toxicity information in this DB will be useful for the development of stage-specific adverse outcome pathways (AOPs) via integration with mechanistic information. The whole datasets of the DB can be implemented in read-across support tools such as the OECD QSAR Toolbox, which will further lead to future integrated approaches to testing and assessment based on AOPs.
Developmental and reproductive toxicity (DART) is one of the important endpoints for assessing the risks of chemical substances, as toxicity results in infertility, miscarriage, stillbirth, birth defects, growth delay, and incomplete development in humans. There are regulatory needs to fill information gaps on potential hazards that chemicals might pose to human health and to identify and implement appropriate heath-protective risk management measures. The latest chemical management policies, including the Registration, Evaluation, Authorization and Restriction of Chemicals in the European Union, the Toxic Substances Control Act in the US, and the Chemical Substances Control Law in Japan, require the toxicological evaluation of marketed but untested chemicals. It is particularly requested that no-observed-adverse-effect levels should be determined for certain susceptible subpopulations (e.g., children, pregnant women).
Although in vivo methods for evaluating DART are standardized, these studies are expensive and time-consuming, making it impractical and impossible to test all chemical substances to which the relevant populations are potentially exposed. Thus, the development of alternative methods to detect high-risk substances is considered necessary. A number of alternative methods, including zebrafish embryotoxicity tests (Brannen et al., 2013; He et al., 2014), the rat whole-embryo culture assay (Webster et al., 1997), and assays to evaluate the development of embryonic or induced pluripotent stem cells (Liu et al., 2017; Luz and Tokar, 2018), can classify most established developmental toxicants; however, most of them are human pharmaceuticals and few industrial chemicals have been validated so far. Conversely, the development of integrated approaches to testing and assessment (IATA) based on adverse outcome pathways (AOPs) is considered beneficial for evaluating the risk of untested chemical substances (Tollefsen et al., 2014; Sakuratani et al., 2018; Yamada et al., 2020; Rovida et al., 2020), although it may be challenging because the numbers of studies on DART available for the assessment and known DART mechanisms are limited.
Although a reliable database (DB) is necessary, DBs including industrial chemicals are not yet sufficient for assessment because the clinical and non-clinical information of human pharmaceuticals was dominant concerning DART. As such, we constructed a new DART DB of industrial chemicals by combining publicly available DBs of Japanese public institutes in which the results of studies conducted according to OECD DART guidelines were compiled. Systematic analyses of the DB provided DART information that will lead to some future AOPs and IATA based on AOP networks.
In total, reports of DART studies by the Japanese Ministry of Health, Labour and Welfare (MHLW) and Ministry of Economy, Trade and Industry under an existing Japanese chemical survey program and Japan challenge program, respectively, were collected from two publicly available DBs: Japan Existing Chemical Database (https://dra4.nihs.go.jp/mhlw_data/jsp/SearchPage.jsp.) provided by the National Institute of Health Sciences and Japan CHEmicals Collaborative Knowledge DB (https://www.nite.go.jp/chem/jcheck/top.action?request_locale=ja.) provided by the National Institute of Technology and Evaluation. Of these studies, 303 studies were conducted according to OECD TG422: Combined Repeated Dose Toxicity Study with the Reproduction/Developmental Toxicity Screening Test (OECD, 1996), and 101 studies were conducted according to OECD TG421: Reproduction/Developmental Toxicity Screening Test (OECD, 2016). The constructed DB, which was named DART NIHS DB, consisted of datasets of the studies, including chemical information; name, CAS No., and structure depicted by the Simplified Molecular Input Line Entry System; test information; animal species and strain; purity of the test substance; vehicle of the dosing formulation; dose levels; dosing route and test guideline; and findings identified by expert reviews (See Supplement file 1).
DB evaluationThe compounds in the dataset of the DART NIHS DB were compared with compounds in other datasets of DART DBs implemented in the OECD QSAR Toolbox (version 4.4). The DART NIHS DB was summarized by the number of studies with developmental and reproductive toxicity findings and the lowest-observed-adverse-effect level (LOAEL) distribution. DART findings in the DB were classified according to the time of occurrence, and are shown in Table 1, as follows: early embryonic development and before including fertility (Stage A), organogenesis (Stage B), and the perinatal period (Stage C). The number of occurrences of each finding was counted for the dataset of the DB.
| Period | DART finding |
|---|---|
| Stage A | No. of mated pairs ↓, No. of copulated pairs ↓, Copulation index (copulated animals/mated animals) ↓, No. of pregnant females↓, Fertility index (pregnant animals/copulated animals) ↓, No. of pairing days until copulation ↑, No. of corpora lutea ↓, Pre-implantation loss ↑ |
| Stage B | No. of implantation scars ↓, Implantation index (implantation scar/corpora lutea) ↓, Post-implantation loss ↑, Gestation length ↑, Malformation in pups ↑ |
| Stage C | No. of dams with live pups ↓, No. of pups on day 0 ↓, Gestation index (dams with live pups/pregnant dams) ↓, Delivery index (pups at birth/implantation scars) ↓, No. of live pups on day 0 ↓, Birth index (live pups at birth/implantation scars) ↓, Live birth index (live pups at birth/pups at birth) ↓, Biased sex ratio, No. of live pups on postnatal day 4 ↓, Viability index (live pups on postnatal day 4/live pups on postnatal day 0) ↓, Body weight on postnatal day 0 or 4 ↓, Nursing activity ↓ |
↓: Decrease, ↑: Increase
All studies in the dataset were conducted in Sprague–Dawley rats. Test substances, among which the purity exceeded 92% for 95% of the substances, were suspended or dissolved in various vehicles including water, corn oil, olive oil, methyl cellulose, or carboxymethyl cellulose solution to maximize the absorption and orally administered via gavage. Comparison of the datasets of DART NIHS DB with those of ToxRefDB, which contains detailed data from studies including DART and repeated-dose toxicity (RDT) endpoints and is comparable in terms of the study quality (EPA, 2018), indicated that only 12 substances were duplicated in these DBs. In addition, comparing with the developmental toxicity ILSI, the Developmental & Reproductive Toxicity (DART) and the Developmental toxicity (CAESAR) in the OECD QSAR Toolbox, there were 8, 8 and no duplicates, respectively. These results suggest that the datasets of DART NIHS DB potentially add a significant degree of depth to the knowledge of DART for compounds that occupy unique chemical spaces.
During the review of individual study reports by experts, it was difficult to clarify whether some findings and their affected doses were attributable to the treatment or they represented effects on dams or offspring. These situations often occur in evaluating the results of DART studies in which the historical background data of the facility were generally referenced when uncommon findings or findings without dose relationships are observed. Because the study report of every dataset is freely referable, confirmation of the study results in the individual reports is recommended in cases in which the findings and their doses critically affect study outcomes. Because this DB will presumably be used to screen the DART of substances, the evaluations during the process of constructing this DB were rather conservative such that even a statistically insignificant change, when accompanied by the associated parameter changes or findings, was considered a treatment-related and toxicologically significant event.
As a result, 171 substances induced DART in studies conducted under OECD TG 422 (123 studies) or OECD TG 421 (48 studies), whereas 233 substances (180 studies under OECD TG 422 and 53 studies under OECD TG 421) had no such effects. Of the 171 substances that induced any type of DART, the numbers of substances which the lowest-observed-adverse-effect level (LOAEL) of DART was ≤ 10 mg/kg bw/day (LOAEL ≤ 10), more than 10 mg/kg bw/day but no higher than 30 mg/kg bw/day (10 < LOAEL ≤ 30), more than 30 mg/kg bw/day but no higher than 100 mg/kg bw/day (30 < LOAEL ≤ 100), more than 100 mg/kg bw/day but no higher than 300 mg/kg bw/day or lower (100 < LOAEL ≤ 300), and more than 300 mg/kg bw/day but no higher than 1000 mg/kg bw/day (300 < LOAEL ≤ 1000) were 11, 19, 34, 52, and 55, respectively (Fig. 1). Notably, more than one-third of the substances that were associated with DART induced changes at doses as low as 100 mg/kg bw/day.
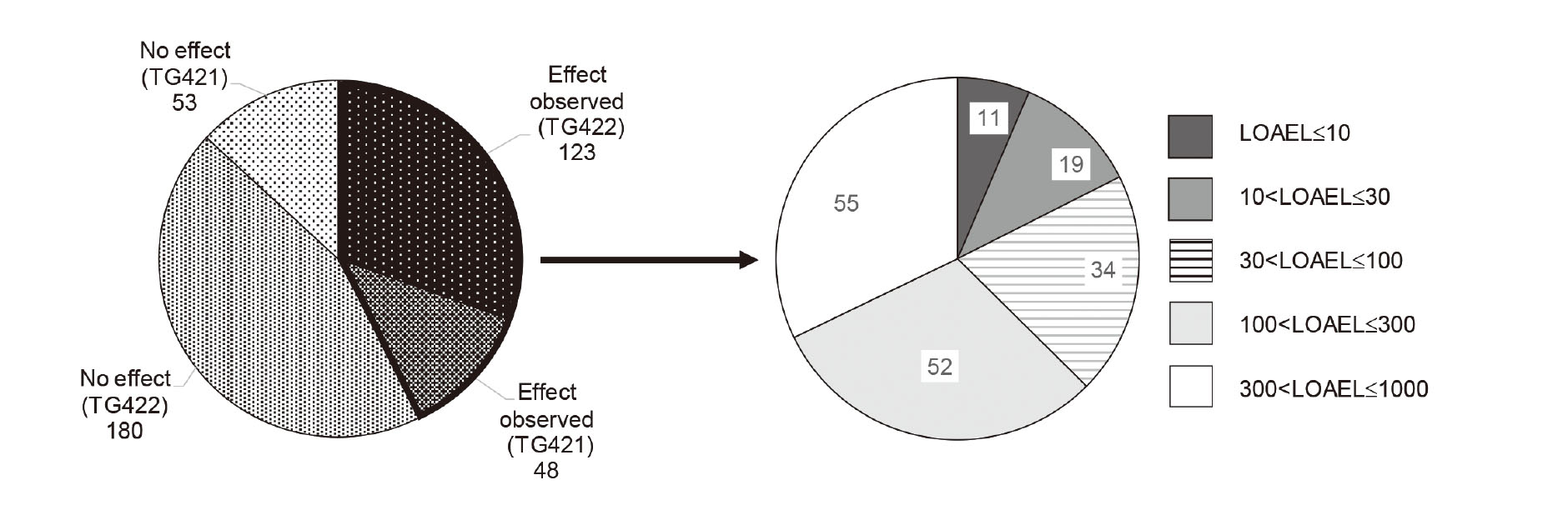
DART NIHS DB summarized by the number of studies with developmental and reproductive toxicity findings and the lowest-observed-adverse-effect level (LOAEL) distribution. The numbers of studies with any effects, which were conducted in accordance with OECD TG421 and TG422, are presented in the left pie chart, and the doses in terms of the LOAEL (mg/kg bw/day) in 171 positive studies are presented in the right pie chart.
In this DB, in which 171 substances induced any DART findings, 49, 59, and 161 substances produced effects in Stages A, B, and C, respectively. Two unclassified substances were associated with only estrus cycle abnormality. The major DART findings in these studies, which were not limited to those that denoted the LOAEL, were as follows: decreases in the fertility index and the number of corpora lutea in Stage A; increases in gestation length and decreases in the number of implantation scar and implantation index in Stage B; and decreases in the number of pups on day 0, delivery index, the number of live pups on day 0, live birth index, viability index, and pup body weight on gestation days 0 and 4 in Stage C (Fig. 2). Because Stage C consisted of periods of the third trimester of pregnancy and lactation, which are comparatively long in humans, it could be noted that 161 of 171 substances could potentially affect development during these periods. For some substances, findings that were not limited to a specified stage and were observed during multiple stages implied that some early changes were associated with later findings. Such detailed evaluations may be requested for certain purposes, and this DB is feasible for these purposes, as the original study reports of each dataset are accessible in the DB.

Findings in DART NIHS DB and their occurrence. The number of occurrences of each finding was counted against the dataset of 404 studies of industrial chemicals in DART NIHS DB. The horizontal axis shows the number of occurrences of each finding. The up and down arrows indicate increases and decreases, respectively. A, B, and C include findings occurring in Stages A, B, and C, respectively.
Because the duration of dosing is identical for studies conducted under OECD TG 421 and OECD TG 422 (i.e., starting 2 weeks before mating and continuing to the lactation period for 13 days), the DART findings in studies conducted under these guidelines are considered comparable. In studies conducted under OECD TG 422, in which the general toxicity endpoints after repeated administration were evaluated in addition to DART endpoints, the LOAEL of DART was compared with that of RDT in females in 123 studies. The LOAEL of DART was comparable to and greater than that of RDT for 43 and 45% of the substances, respectively (See Supplement file 2). The LOAEL of DART was lower than that of RDT for 15 substances (12%). Of these, five substances exhibited significant DART findings at doses of ≤ 50 mg/kg bw/day. They are considered more specific to DART because the LOAEL of DART was distinguishable from that of RDT, and the effects of RDT were negligible. The chemical structures of these substances and toxicity findings in the studies are presented in Table 2. These substances were as follows: 2,2'-dichlorodiethyl ether, which induced increases in post-implantation loss and decreases in delivery and birth indices, suggesting effects in Stages B and C; cyanochlorobenzene, which induced body weight decreases on days 0 and 4, suggesting effects in Stage C; 4-methylpyridine, which induced estrus cycle abnormality, increases in gestation length, and decreases in birth, live birth, and viability indices, suggesting effects in Stages B and C; benzyl salicylate, which induced increases in gestation length and malformation and decreases in gestation, delivery and viability indices, the number of live pups, and pup body weight on day 0 (Igarashi et al., 2018), suggesting effects in Stages B and C; and 2-ethylbutyric acid, which induced decreases in the number of live pups on day 0, birth index, live birth index, number of live pups on day 4, and abnormal delivery, suggesting effects in Stage C. Neither similar chemical structures nor common toxicological or biological characteristics were found among these substances. Individually, it was reported that chloroacetic acid, a possible metabolite of 2,2'-dichlorodiethyl ether, affected fetal development in rats (ATSDR, 2017; EPA, 2004). Benzyl salicylate is expected to be degraded to salicylate. The metabolite has inhibitory effects on histone deacetylase (HDAC), which is known to be associated with developmental toxicity (Menegola et al., 2006; Di Renzo et al., 2008). 2-Ethylbutyric acid is structurally similar to valproic acid. However, the biochemical profile related to developmental toxicity endpoints was not similar to that of valproic acid (OECD, 2019). Overall, the biological and toxicity information of the five substances was so limited that the etiologies of the DART-specific findings could not be fully explained. Nevertheless, examining DART endpoints is essential for the hazard identification and risk assessment of chemicals. Moreover, in our preliminary analysis, no specific structural relations to DART were identified in this DB excluding several perfluorocarbon chemicals that induced body weight decreases in neonates and actually induced changes in Stage C. However, a more comprehensive analysis with the expanded datasets and integration of relevant knowledge could allow for the identification of common chemical structures associated with DART.

DART NIHS DB is valuable, as the included datasets consisting of every change and the dose of the observed effects were created directly from publicly available toxicity study reports, which were conducted under the designated guidelines. The utility of the DB in predictive toxicology is comprehensively illustrated in Fig. 3. Benzyl salicylate, whose specific DART was stated previously, may fall into the category of salicylates causing HDAC inhibition, when it follows the illustrated flow and is consulted. The DB can be implemented in support tools such as the OECD QSAR Toolbox for grouping chemicals into categories and filling the data gap of endpoint of inquiry substances via read-across (Dimitrov et al., 2016). Making this DART DB available together with existing DART DBs in a single tool increases the convenience and probability of finding analog substances. It is notable that information about empirical metabolites and plausible metabolites by the metabolism simulator is available in the QSAR Toolbox. This feature allows users to group tested and untested substances that produce the same toxic metabolites into categories.
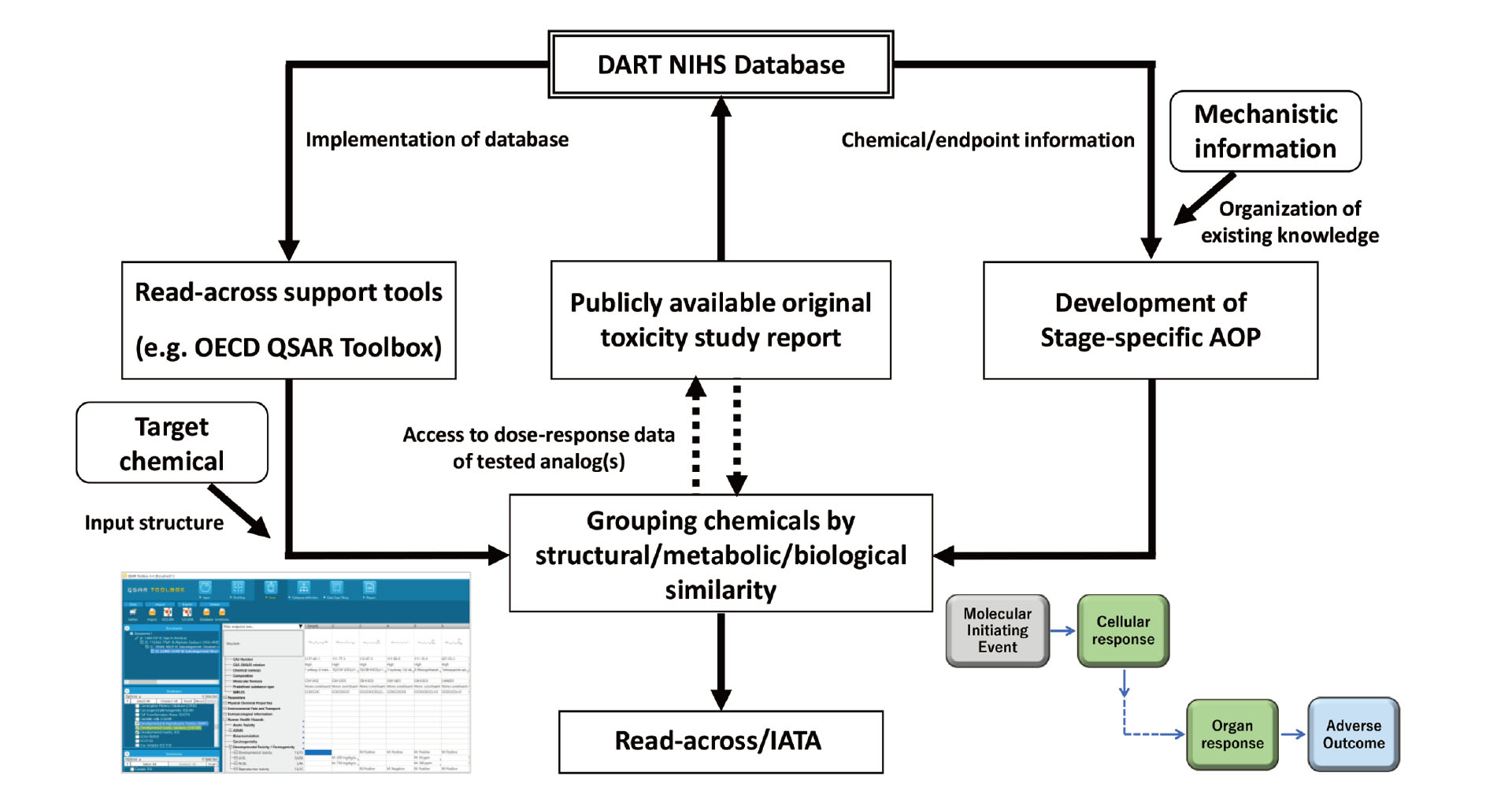
The utility of DART NIHS DB in predictive toxicology. DART NIHS DB can be implemented in read-across support tools such as the OECD QSAR Toolbox and used in conjunction with its existing databases (DBs) to group chemicals into categories. Moreover, chemical and endpoint information in the DB will be useful for the development of stage-specific adverse outcome pathways (AOPs) via integration with mechanistic information. In addition, when performing read-across, if necessary, the original test data of similar substances can be referred to confirm the degree of change in the target endpoint and dose–response relationship. DART NIHS DB can contribute to the development and improvement of read-across/integrated approaches to testing and assessment (IATA).
The DB will also be utilized to develop AOPs corresponding to the period and timing in which a respective DART finding is observed, specifically Stages A, B, and C. To develop stage-specific AOPs, information about both chemical structures and biological characteristics including endpoints (DART findings) and the timing of occurrence is fundamental. Organizing existing knowledge including literature information on the toxicological mechanisms of the substances will support the development of AOPs. Biological activity data corresponding to key events of the AOP will increase the confidence in grouping substances based on similar biological activities.
IATA based on AOPs will be generated by combining all relevant information of the inquiry and analog substances. When data are read across from analog substances in the DB, it is possible to refer to original study data of the analogs to reevaluate the toxicity based on the severity of endpoint changes and their dose responses. This highly reliable and transparent DB will be essential for read-across because the reliability of read-across primarily depends on the selection of suitable analogs of an inquiry compound and the quality of the toxicity data. The datasets will also serve as training and validating predictive models for DART (Sipes et al., 2011; Wu et al., 2013; Hisaki et al., 2015). In conclusion, assessments of the toxicity DB containing DART information of a variety of substances will be indispensable for predictive toxicology and IATA based on AOPs.
This study was supported by MHLW under Health and Labour Science Research Grant (H30-Kagaku-Sitei-005). The authors thank Ms. Mika Inoue for the technical assistance in manuscript preparation.
Data availability statementThe dataset for this study is available at NIHS homepage (http://www.nihs.go.jp/dra/NIHS_DBs.html).
Conflict of interestThe authors declare that there is no conflict of interest.